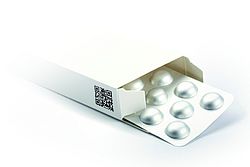
Tampering Directive – European Directive on Falsified Medicines
09.10.2012 - Recycled Cartonboard, Virgin Fibre Cartonboard
The European Commission published the Falsified Medicines Directive 2011/62/EU in July 2011 as part of a package of measures to effectively combat the falsification of medicinal products. This will allow wholesalers and pharmacists to determine if the medicine is genuine and if the outer wrapping of has been tampered with.
MMK 2D qualities for more security
More and more medicines are now being falsified. One in every ten medicines ordered on the Internet is fake, according to the World Health Organisation (WHO). Legislation relating to transparent distribution chains and state-of-the-art security techniques is important in the fight against the increase in falsified medicines.
EU calls for two security features
The security features are separated into two groups. The first consists of ‘individual identification marks’ such as serial numbers and DataMatrix codes. These are primarily used by wholesalers and pharmacists to check the authenticity of medicines and identify individual packets. In order to be read correctly in accordance with ISO 15415, the cartonboard qualities require an optimised coating to be dry and smudge-proof in less than half a second.
The MMK qualities Excellent Top 2D, MCM 2D and Supra 2D were developed to master this special requirement. The 2D emphasises the specific suitability for the use of DataMatrix codes.
The second new group consists of anti-tamper features (tamper proof evidence). There are several options: Stickers, adhesive seals, cellophane wrapping and perforated openings that can be used, depending on the manufacturer’s specifications, to determine if the outer wrapping of the medicine has been tampered with. There are to be no requirements governing the various options that could be implemented by the packaging manufacturer. All of the mechanisms can therefore be considered equal and may be used simultaneously as long as they ensure that it is easy to inspect the outer packaging in day-to-day operations for signs of tampering. Examples include closures that have to be torn open to access the product or seals. There are also plans to introduce a common, EU-wide logo to identify legal online pharmacies.
All EU Member States will start applying these measures from 2 January 2013* and they will become binding from 2016.
*Does not include Belgium, Italy and Greece